A Look at How COVID-19 Vaccines Get Approved
Over 500 million doses of the COVID-19 vaccine have been administered in the United States since January of 2021 and have been credited with saving millions of lives. However, many across the country are still hesitant to get the vaccine or decline it entirely.
If you are still trying to decide whether or not to get the COVID-19 vaccine or get a booster, a look at this process might help. Here are the steps the COVID-19 vaccine underwent to get from a pharmaceutical lab to a shot in a patient’s arm.
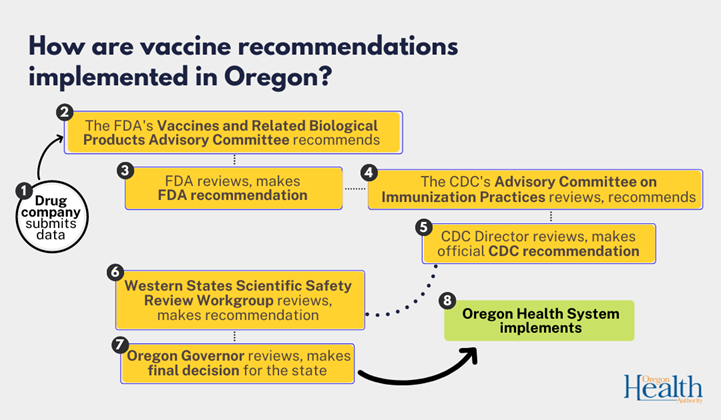
Before we could offer the vaccine in our clinics, seven different advisory groups reviewed and approved the vaccine to ensure it is as safe, effective, and necessary as possible. These detailed reviews took place one at a time, and each advisory group had to approve the vaccine before it could proceed to the next review group. If one group did not recommend the vaccine, the process would have stopped and not continued.
Only after these seven advisory groups approved the vaccine’s use could it be made available to the public. These reviews take place at both a national and state level.
The full review process for a new vaccine typically takes a couple years. However, due to such high demand for a COVID-19 vaccine, this review process was sped up, and the vaccine was approved within months of its development. Review groups worked around the clock to get the vaccine approved and distributed as soon as possible.
Every vaccine available to the public goes through this review process. You can feel confident each vaccine has been thoroughly scrutinized before it is approved for public use.
NHC alone has administered thousands of vaccines for COVID-19, influenza, and other serious illnesses, which have prevented many from contracting serious infections.
As concerns of an impending wave from a second Omicron variant strain heighten, it is even more important that those who are not vaccinated receive their COVID-19 immunizations. The COVID-19 vaccines remain our best tool to keep you from getting a severe or extended illness.
NHC providers, like myself, are happy to answer any questions you may have about vaccines and are available to talk through hesitations and concerns with you. Our priority remains your health and safety, and we will continue to provide COVID-19 vaccines for those who need them.
Sincerely,
Dr. Ann Tseng, CMO, MBA
